
Recently, the team led by Researcher Yang Hui from the Shanghai Advanced Research Institute of the Chinese Academy of Sciences and Ningbo Zhongke Kechuang have made important progress in the research and development of iridium-based catalysts for the anode of proton exchange membrane water electrolyzers (PEMWE). Traditional IrO₂ catalysts follow the adsorption evolution mechanism (AEM), which is restricted by the linear relationship and it is difficult to break through the intrinsic activity. Lattice oxygen mechanism (LOM) By directly utilizing lattice oxygen to participate in the reaction, the energy barrier limitation can be broken, but it will cause the loss of lattice oxygen and weaken the Ir-O bond. The team proposed a sulfur-mediated IrO2 catalyst (IrO2/S), which switches the OER pathway from the traditional AEM to the LOM mechanism. At the same time, the lattice distortion and unsaturated Ir-O coordination in IrO2/S produce oxygen non-bonding states, which act as electron sacrificial agents to preserve the Ir-O bonds in the LOM-dominated OER process. IrO2/S exhibits excellent catalytic activity (1.769 V, 2.0 A cm-2) and stability (16.6µV h-1@1.0 A cm – 2) at low Ir dosage (0.3 mg cm-2). The related results were published in Advanced Materials on June 23 under the title of "Sulfur-Doped IrO2 Enable Pathway Switch to Lattice Oxygen Mechanism with Enhanced Stability for Low Iridium PEM Water Electrolysis".
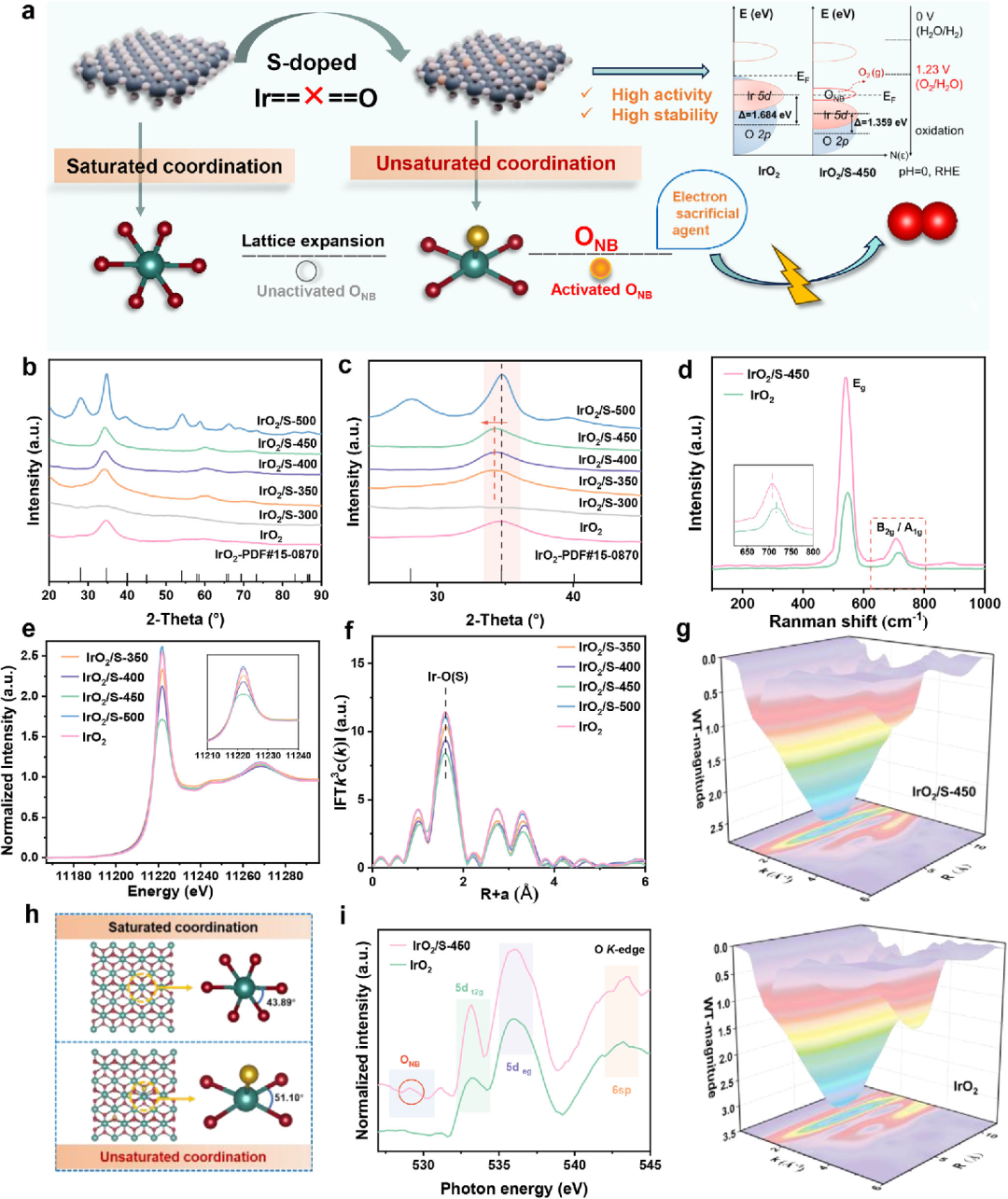
Fig.1 Schematic diagram of IrO2/S catalyst design strategy and related element analysis
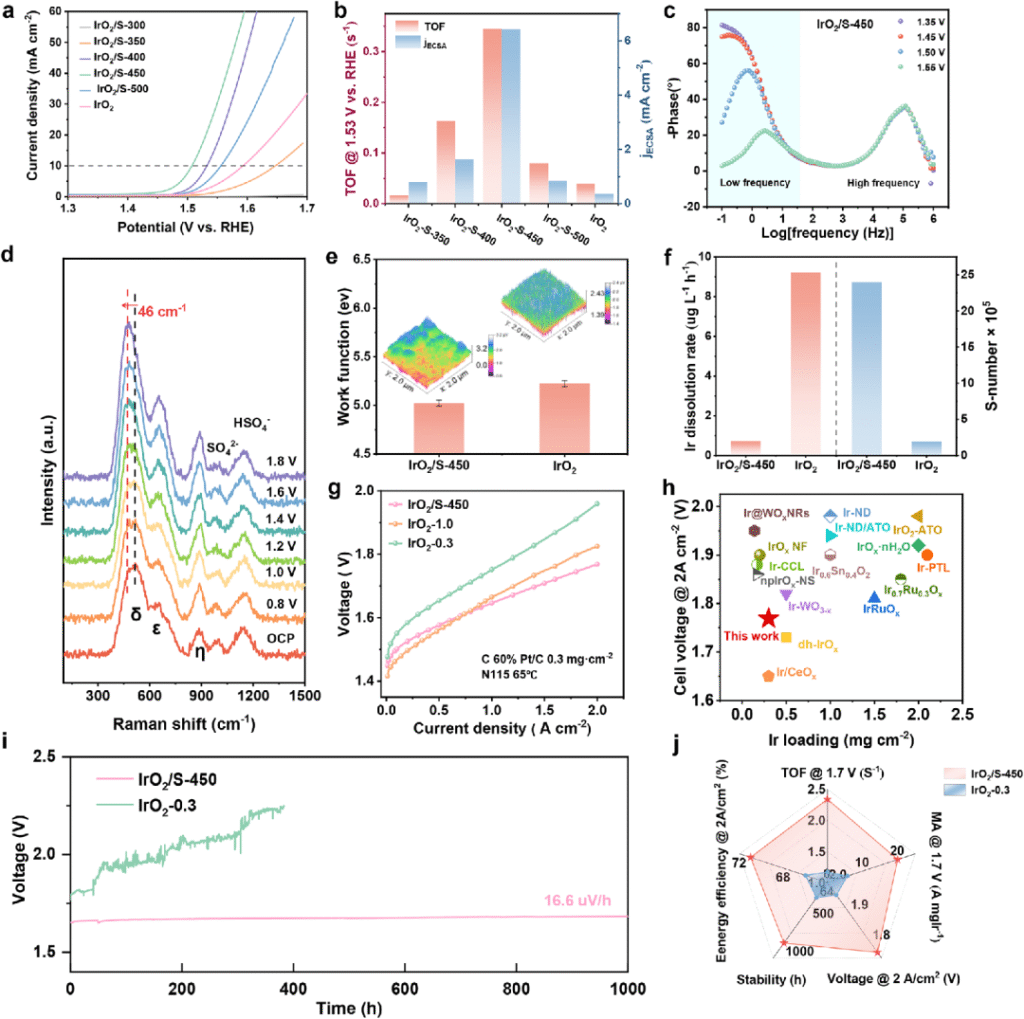
Fig. 2 Electrochemical performance of IrO2/S catalyst and practical application verification of membrane electrode
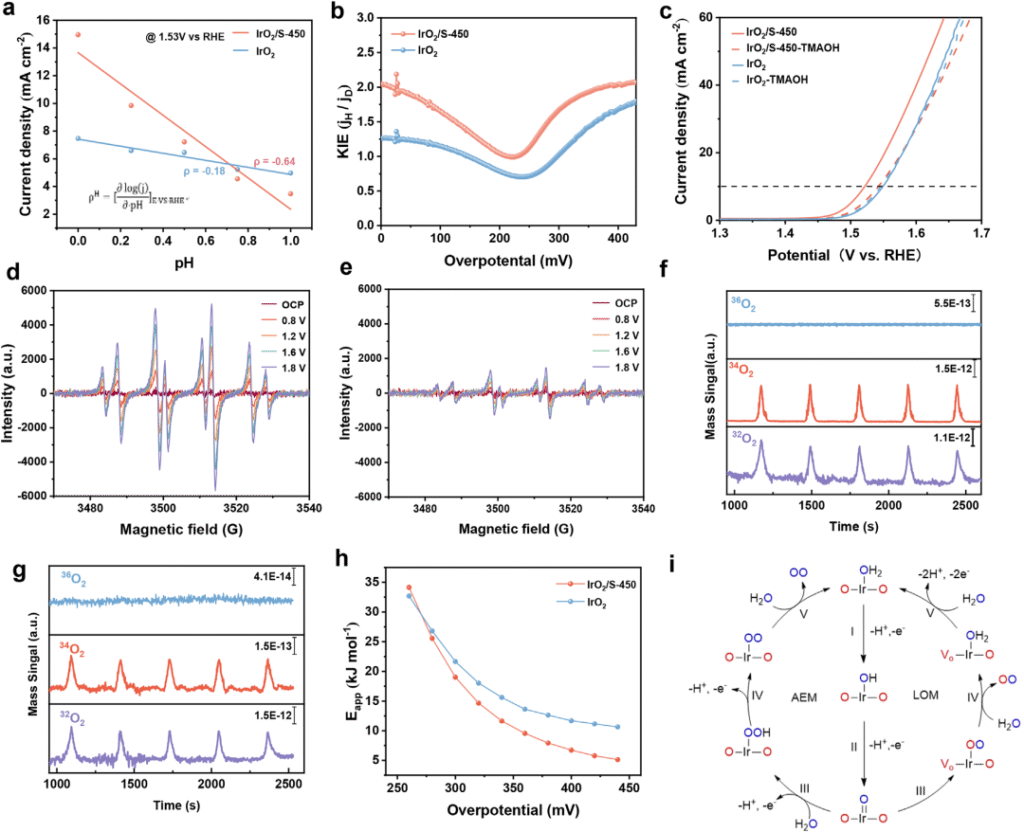
Fig.3 Exploration of reaction mechanism of IrO2/S catalyst
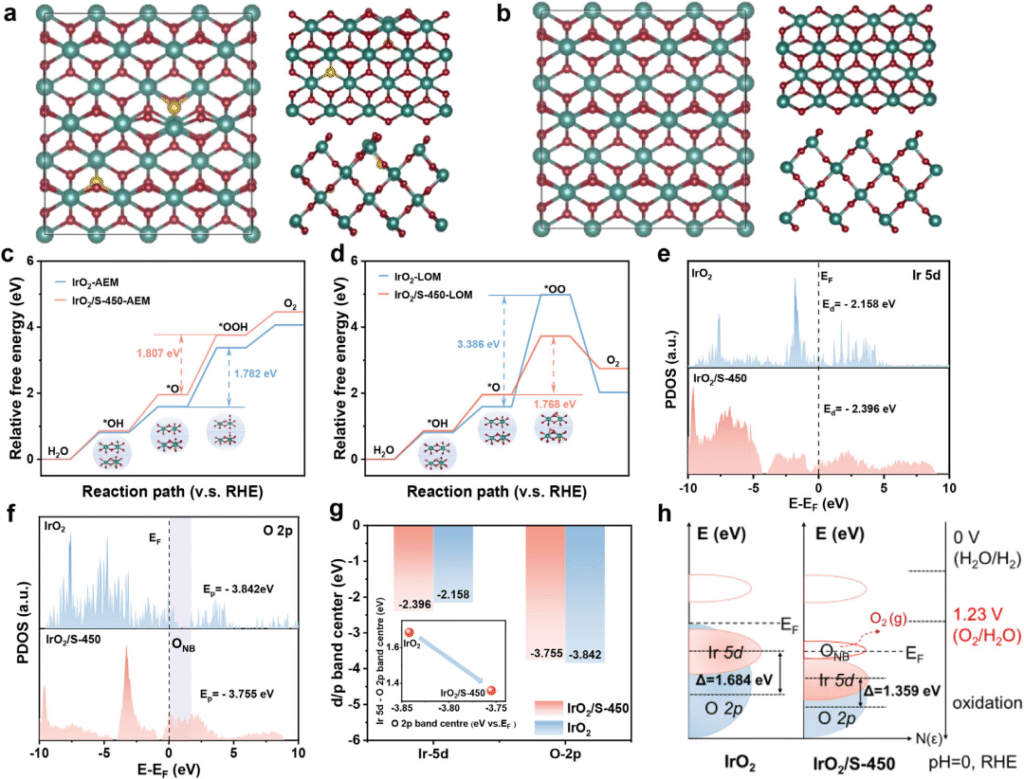
Figure 4 Theoretical study on the activity and stability related properties of IrO2/S catalyst
In summary, our proposed S/IrO2 OER catalyst exhibits excellent performance and stability in PEMWEs, with a cell voltage of 1.787 V at 2.0 A cm-2 and stable operation for 1000 h at 1.0 A cm-2 at low Ir dosage. S doping can not only switch the OER pathway on IrO2 from the traditional AEM to LOM, thus breaking the linear relationship and improving the intrinsic activity, but also produce ONB bands that preserve the Ir-O bond order, thereby improving the stability of the LOM pathway. Overall, this study provides a rational strategy for the design of OER catalysts with low Ir dosage and draws a blueprint for the development of high-performance electrolysis technology in line with global decarbonization goals.
Paper link: https://doi.org/10.1002/adma.202507560



